Blog
See all posts20/05/2022 - Journal Club
A Systematic Review on Bioethics in Proteomics
The moment to begin exercising control over the rules and regulations that will bind us tomorrow is soon. The time to begin thinking and talking about them is now.
In this month’s Journal Club, we have decided to step away from technical topics such the latest advances in proteomics methodologies. Instead, we will approach a more “philosophic” topic, that of bioethical principles in the context of (clinical) proteomics. It may not be a topic that is as adrenaline-inducing as the latest and coolest scientific discoveries of our days. None the less important, bioethics is a highly relevant subject that concerns all scientists in their duty to conduct socially responsible research (Resnik & Elliott, 2016). The gallery picture for this post is taken from the cover of the American Journal of Bioethics, December 2021, Volume 21, Number 12.
Introduction
In the article we will present here, entitled “Ethical Principles, Constraints, and Opportunities in Clinical Proteomics” (Mann et al., 2021), the authors introduce the notions of bioethical principles and how they relate to proteomics research. They then present the results of the systematic review of the literature they performed on the principles of bioethics in clinical proteomics and finally, they provide their own perspective on some of these topics.
Info box: Systematic review
Systematic reviews are reviews that aim to answer a clearly defined question. To this end, they use systematic and reproducible methods to identify, select and appraise all relevant research on the topic of interest. Further, they collect and analyze the data presented in the studies that are included in the review. As systematic reviews strictly adhere to scientific design, there are strict and well-defined guidelines to frame their methodology, such as the PRISMA guidelines (Moher et al., 2009).
Evidently, ethical concerns and responsibilities are not novel in biological sciences. Such issues have often been addressed and taken into account in the past. The field of genomics with its vast expansion during the last two decades constitutes an excellent example where bioethical questions have been extensively addressed and good practices established (for example see Kalia et al., 2017). During the recent advent of proteomics technologies and while the field has focused on developing its foundations, bioethical issues have so far been neglected, but it is now time to consider and discuss them in response to the increased capabilities of proteome analysis. To support this very argument, Mann et al. refer to their work on plasma proteomes highlighting how they can be reindentified based on protein expression levels or variant peptides, and published in an accompanying article (Geyer et al., 2021).
Core Principles
In the first section of their review, the authors introduce the core principles of bioethics. Specifically, they identify and center the rest of the article around four main principles, for which they provide some specific translations to the scientific research context (Fig 1). As argued by the authors, while these issues may seem too theoretical and abstract, they should directly affect the decisions on what data to collect, how to analyze them and how to disseminate the results of proteomic research.
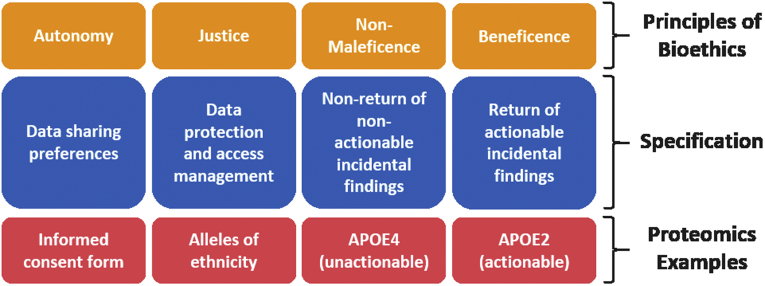
Nonmaleficence is, in simple terms, the (moral) duty to not cause harm. A concrete example that the authors provide, is in the context of incidental findings, whereby a result of unknown significance or that indicates a certain predisposition to a condition can emerge from a proteomics analysis.
Beneficence could be easily thought of as the opposite of the aforementioned (non)maleficence, i.e. the idea of benefiting others. Staying within the same example of incidental findings, reporting them to the individuals that are concerned (directly or indirectly) could prove beneficial if such findings are found to contain information that can improve diagnosis or treatment.
The principle of justice is related to fairness and equality, and a prime example most of us are probably familiar with is the application of FAIR principles to our data, but other related topics mentioned here by the authors are the disclosures of conflicts of interests and the need for representative databases.
Being autonomous is the ability to choose laws (nomos in greek) for oneself (auto in greek). The authors cite the philosopher’s Immanuel Kant work on the matter, who argued that autonomous agents must also respect the autonomy of others and that this forms the basis of human dignity. In terms of biological research, a concrete manifestation of autonomy is the implementation of informed consents filled and signed by study participants.
Experimental procedure
The authors aimed to collect all existing literature that one way or another deals with these bioethical principles in the context of proteomics. They used specific methods for literature search that are described in detail in the publication, which resulted in 365 unique articles. From there, filtering was applied to remove articles if (a) the mention to the four bioethical principles was considered too limited or peripheral, (b) proteomics was not distinguished from genomics or (c) it was written in a language other than the ones with which the authors have native competencies. As a final result, 16 studies were included for further analysis. These were processed by a trained bioethicist in consultation with a clinical proteomic scientist, who, using a mixed-model approach, aimed to extract data (in this case bioethical issues) and carry out a thematic analysis. Ultimately the authors grouped the identified bioethical issues into 10 themes.
Systematic Review Results
As we walk through the informative blog, now we understand what a systematic review is (summary of all the research on a specific topic based on certain criteria). The review includes 16 total articles. Further, we leap to the results. Talking about ethical issues, we remark that they are relevant and required in the field of clinical proteomics. Based on the systematic review, ten unique categories entitled ‘theme’ are introduced to emphasize the importance of bioethical issues in clinical proteomics (Fig 2). The categories are mentioned in detail below.
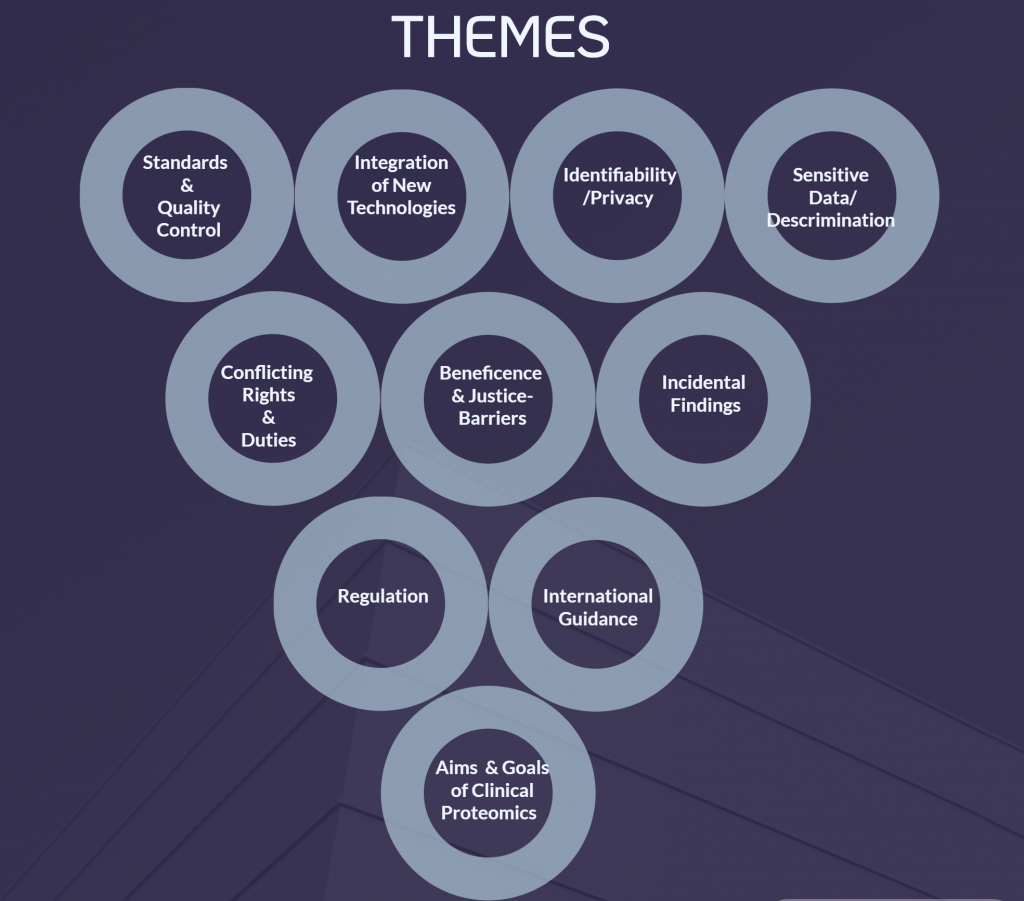
Theme 1 – Standards and Quality Control: The considerable effects of variation in all stages of the proteomic workflows, offers an urgent need to initiate and establish conventional operating proceduresinclusive of consistent quality checks. The proteomic workflows mentioned in the literature comprises study design, preanalytical factors, sample collection, storage, and shipping condition. Each workflow mentioned by three authors is implemented by them. With the help of these checks, reproducibility, interoperability, and cooperation among proteomics research labs and allied fields are expected to flourish. Talking about the literature review performed by authors, they present an additional check, an ethical perspective to the technical issues evolved. Therefore, ethical view comes into play while contemplating trust and actual expectation than reality by participants, patients, and the research community at large.
Theme 2 – Integration of New Technologies and Related Fields: Author mentioned about the need to be updated regarding technology and scientific advances. Such instance is an article proposed the usage of blockchain technologies to be cautious about transparent and secure data access management (Boonen et al., 2019). Additionally, emphasized on the benefit and utilization of linking proteomics with the increasing metadata.
Theme 3 – Identifiability/Privacy: The author proceeds with briefly communicating about proteomic profile,the possibility of uniquely identifying individuals. The early studies remarked this proteomic profile as a hypothetical possibility of human tissue or the databank studies. For example, with the help of hair samples, prominent keratin proteins can assist in differentiating individual profiles, implying to utilize the research in forensic use as well (Laatsch et al., 2014). Also, it was a confirmed information that identifying individuals and the ethnic background can be derived from hair proteome. The similar illustration can be performed for plasma studies with the help of plasma proteome.
Theme 4 – Sensitive Data/Discrimination: The most important ethical concern with respect to usage ofindividual personal sensitive data at times can be used to humiliate individuals. Such occurrences have been reported by Geyer et al, revealing the potential of proteomic profiles, which can be utilized to reveal pregnancy, weight, ethnicity, gender, and allele status of the individuals. But looking to an optimistic outcome, such information can be used for knowing about the family members of a particular patient.
Theme 5 – Conflicting Rights and Duties: This category reveals the important aspect of introducing rights and duties, such as duties expected by the patients may be a conflict with the duties expected by the scientific community. For example, the author talks about the data anonymization, which means right to remove or encrypt the dataset from the data which reveals the personally identified information. This could be used to facilitate the privacy concerns of the patients but can also be a serious concern for the research that includes data correlation. The author mentions about the seven studies emphasizing on the importance of possible conflict of interest between several stakeholders in proteomics (clinical), emerging from intellectual property (IP) protections. IP protections serves best to motivate, execute scientific and innovative ideas, but due to present high level of protection, researchers may find it difficult to access required data or materials for the ongoing research (Lennart & Vizcaíno, 2017).
Theme 6 – Beneficence and Justice-Barrier: The author presents the problems faced by researchers in low/middle-income countries urged by IP protections. Nine studies revealed profit sharing or data sharing as an ethical issue, specifically talking about the databases that are not presenting the global human diversity. Moreover, key highlights regarding the inequitable distribution of benefits in proteomics, concluding to inclusion of financial support from organizations for financial expenses and urgent need of scientific experts.
Theme 7 – Incidental Findings: The author introduces four articles pointing out to the incidental discovery in case of plasma proteomics, functional for medical or social services. In addition, findings, and incidental discoveries from re-evaluation of authenticated pre-existing datasets can be advantageous for advancing research (Boonen et al., 2019). A question arises more on how to maintain, store databases, or look for updated information regarding ongoing research in proteomics and make it available to peer.
Theme 8 – Regulation: The author focuses on the issue of regulation for important national and regional rules & regulations. Mostly talking about informed consent, presented as actual solution. But due to several issues emerging pertaining to research in proteomics such as existing regulation is not enough to acknowledge the issues related to interoperability, efficiency and tasks expected in case of patients and scientific advancements. There is an urgent need to address the issues and discuss about it.
Theme 9 – International Guidance: The author highlights the lack of guidance and simultaneously need for international guidance on the ethical issues. The issue is so prominent that it was mentioned by most of the reviewed articles. Additionally, as a solution stresses on the importance of international collaborations on ethical and scientific concerns.
Theme 10 – Aims and Goals of Clinical Proteomics: The author concludes with researchers talking about guidance in the field of proteomics (clinical) should go beyond the rules & regulations to acknowledge fundamental issues focusing more on goals and funding the projects. In addition to it, how clinical proteomics help us, the human race to overcome health challenges.
Additional perspectives
With this section of the blog, the authors represent their own perspective with respect to bioethical possibilities of clinical proteomics. It starts with talking at length about the standards and study designs in clinical proteomics.
Standards and Study Designs
This section of the blog covers three major concerns from authors own outlook.
1) Medical potential: First point outlines the importance of medical potential of clinical proteomics, which depends upon determination of two groups based on differing health or disease states. To find the inference from these techniques, it mostly depends upon study design, statistical power, thus depicts the differentially express or regulated proteins. Talking about plasma proteomics in this regard, the prototype is to analyze the small number of samples thoroughly. But in case if the data is not enough to perform an analysis, each step performed is futile. For such rationale, the author suggests rectangularly shaped study blueprint than triangularly shaped study blueprint, meaning various large samples are analyzed parallelly to enhance statistical power and significant research.
2) Bias: The author introduces an important viewpoint related to biasness. The recent analysis regarding psychological literature recognizes 34 researcher’s choices in laboratory-based study prototype, where conscious or unconscious bias may arise.
3) Open and Transparent science: The key highlights from making the data availability to all the proteome researchers is an important aspect in open and transparent science leading to FAIR accessibility to the data. This will allow researchers from all corners to re-evaluate and reuse data sets, therefore improve the outcome and benefit everyone.
Overcoming Challenges from Clinical Genomics
Talking about Challenges from clinical genomics, general privacy and health data regulations are significant. As individuals may be discriminated based on health data or demography, to address such issues several regulations have been designed. For example, General Data Protection Regulation (GDPR) and the US Health Information Portability and Accountability Act are implemented to overcome some challenges in the domain. In addition to it, US Health regulations strongly believes in informed participant consent. But also comprises of broader research exemptions as solution to this, such as data minimization and pseudo randomization approach to process the data without consent.
Moving ahead and talking about Individual research and incidental findings, acknowledging actionable and unactionable information is considered of utmost importance, from ethical point of view. According to the reviewers, actionable information should be returned to the individual or their health care provider and non-actionable information should not. Here, actionable information implies to specific actions taken to overcome health condition. And with this the question remains answered what should be done with the incidental findings, should include them in the scientific databases or in health registries.
Another important aspect related to genomics and proteomics rises, when concerned about data reuse or re-evaluate the pre-existing data where authors are not in reach to be contacted. As an answer to this issue, the authors suggest consent for the return of the data, or its storage could be partially informed.
Justice is another situation to deal with. For instance, while talking about polygenic risk scores (number that estimates the effect of genetic variations on individual phenotype), it is more precise for European ancestry than any other ethnicity. This is proved with the availability of 79% of reference genomes from caucasian ancestral lines despite only contributing to 16% of the human population. Moreover, producing demographically representing datasets and Caucasian samples leads to bias in the analysis. This could be overcome with one of the factors such as the financial support to low-to-middle income countries so that such experiments can be carried out. Collaborations act as a boon for such research activities.
Therefore, a strong need of financial assistance somewhere overcomes the access to the benefits from clinical proteomics and additionally in solving issues of injustice.
Benefits of Clinical Proteomics
Clinical proteomics plays a vital role in collecting the phenotypic information for the researchers and patients at large. This aspect has a potential to advance and fully explore the biomedical domain if bioethical concept is understood and implemented correctly. Proteomic profiling being a significant aspect to clinical proteomics, assists with several advantages such as providing the adequate information regarding the environmental and endogenous effects on health. Such information can be utilized to impart reasonable health information, therefore advancing in the biomedical field, and sharing its relevance in the real-world.
Clinical Proteomics is inclusive of neglected factors such as lifestyle of the individuals along with sociocultural and biotic & abiotic determinants of the health.
The implementation of such approach is witnessed in one of the studies of scientific wellness. In the study, multiomic (proteomics, dense dynamics personal data) data was profiled in order to recognize putative biomarkers and coming up with actionable health advice thus leading to improvement in the measured biomarkers among the population.
Conclusion
With this, we come to summarize the discussion in view of characterizing 10 ethical themes from the 16 included studies. Also, we have briefly discussed about the challenges and solution to overcome barriers in the fields of genomics and clinical proteomics. And provide an additional perspective from authors point. Majorly, an important aspect of not only taking proteomics scientists, budding proteomic researchers into account, collaborating physicians but also engage patients, their advocates, or their organizations (as seen in the field of genomics). This broadens the viewpoint and proves an effective measure for the patients undergoing health issues. Additionally, to persevere in the field of clinical proteomics, the author suggests that we should aim for responsible self-regulation. Specifically, self-regulation based on values is generally proved to be more helpful and authorized. Thus, the experience of genomics reveals the relevant discussions about ethical issues existing in clinical proteomics that can be advantageous to different professions like social scientists, lawyers, ethicists, and humanists. To sum up the discussion from where we started “The time to begin thinking and talking about them is now”.
If you have made it this far, despite the theoretical depth of the subject, we would love to hear your thoughts in general on bioethics, or in particular on a subject that we touched upon in this post. For example, do you think that, as scientists, we should only be concerned with the hard facts of reality, or should we remain attentive to subjective things like the ethical norms discussed here?
Do you think non-actionable findings are insignificant and should not be reported? Do you often talk about such findings in your current research groups?
References
Boonen K., Hens K., Menschaert G., Baggerman G., Valkenborg D. & Ertaylan G., Beyond genes: Re-identifiability of proteomic data and its implications for personalized medicine. Genes 682 (2019).
Geyer, P. E., Mann, S. P., Treit, P. V. & Mann, M. Plasma Proteomes Can Be Reidentifiable and Potentially Contain Personally Sensitive and Incidental Findings. Mol Cell Proteomics 20, 100035 (2021).
Kalia, S. S. et al. Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): a policy statement of the American College of Medical Genetics and Genomics. Genetics in Medicine 19, 249–255 (2017).
Laatsch, C. N. et al. Human hair shaft proteomic profiling: individual differences, site specificity and cuticle analysis. PeerJ 2, e506 (2014).
Martens L. & Vizcaíno J. A. A golden age for working with public proteomics data. Trends in biochemical sciences 42.5, 333-341 (2017).
Mann, S. P., Treit, P. V., Geyer, P. E., Omenn, G. S. & Mann, M. Ethical Principles, Constraints and Opportunities in Clinical Proteomics. Mol Cell Proteomics 100046 (2021).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339, b2535 (2009).
Categories
Latest posts

08/05/2024 - Journal Club
Cross-Border Collaboration: Enhancing Peptide Identification with MS2Rescore and MS Amanda

08/09/2023 - Journal Club
Exploring Cellular Complexity: Unveiling Single-Cell Proteomics

23/08/2023 - Journal Club
Modeling Lower-Order Statistics to Enable Decoy-Free FDR Estimation in Proteomics
